With access to anonymised real-world evidence through multicentre data analysis, we provide first-class data quality and seamless integration with existing sources.
Our technology enables its users to structure healthcare data from various sources into the OMOP Common Data Model. This innovation allows life science partners to use 100% anonymised patient data, breaking down barriers between healthcare and life science.
Simple & Fast Data Evaluation
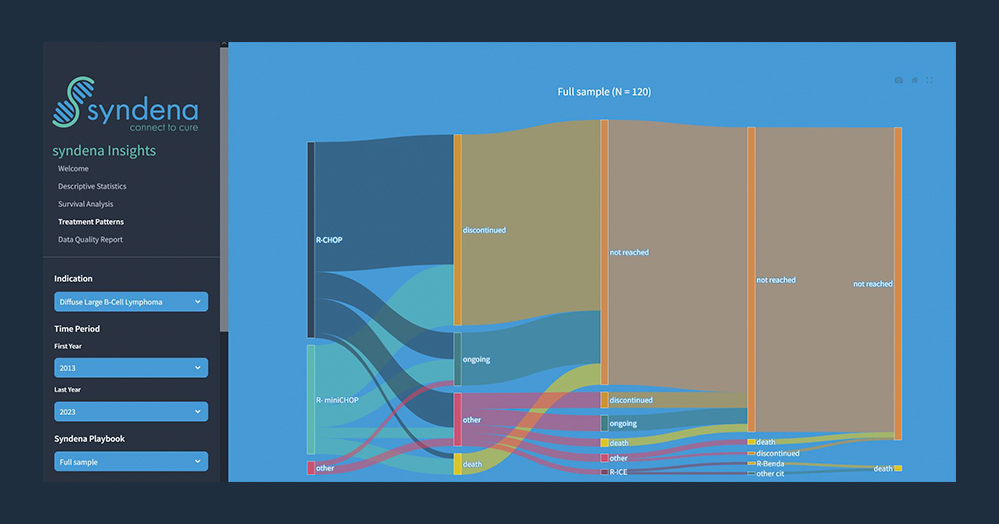
Our streamlined data evaluation process facilitates the rapid extraction of valuable insights from a wide range of clinical research data, including real-world healthcare data and patient reported outcome measures. Researchers can analyse actual anonymised treatment data to gain insights into trends and disease patterns. This helps them to develop optimal solutions for a wide range of conditions.
High-Quality Data
syndena meets the highest standards of clinical data quality through structured real-world evidence (RWE) and the seamless integration of various data sources. Our experts are always working to advance syndena insights, ensuring our data quality is unsurpassed. By employing rigorous plausibility checks, consistency checks and error minimisation processes, our team upholds the highest standards for reliable real-world evidence in healthcare and clinical research.
Collaborative Use of Data
Our collaborative approach supports the efficient use of healthcare data to develop custom-fit solutions. We focus on achieving interoperability between existing data sources and technical systems to enable a seamless data exchange. syndena insights efficiently enables collaborative multicentre data analysis with other research centres and life science partners.
Data Protection: GDPR-Compliant
syndena complies with strict ethical guidelines and data protection standards. Our system is developed and hosted in Austria and Germany. All patient data remains within the clinical environment to comply with medical data protection regulations. Before data can be used collaboratively by other research centres or life science partners, all data is fully anonymised, ensuring it cannot be attributed to individuals.

How Does syndena insights Work?
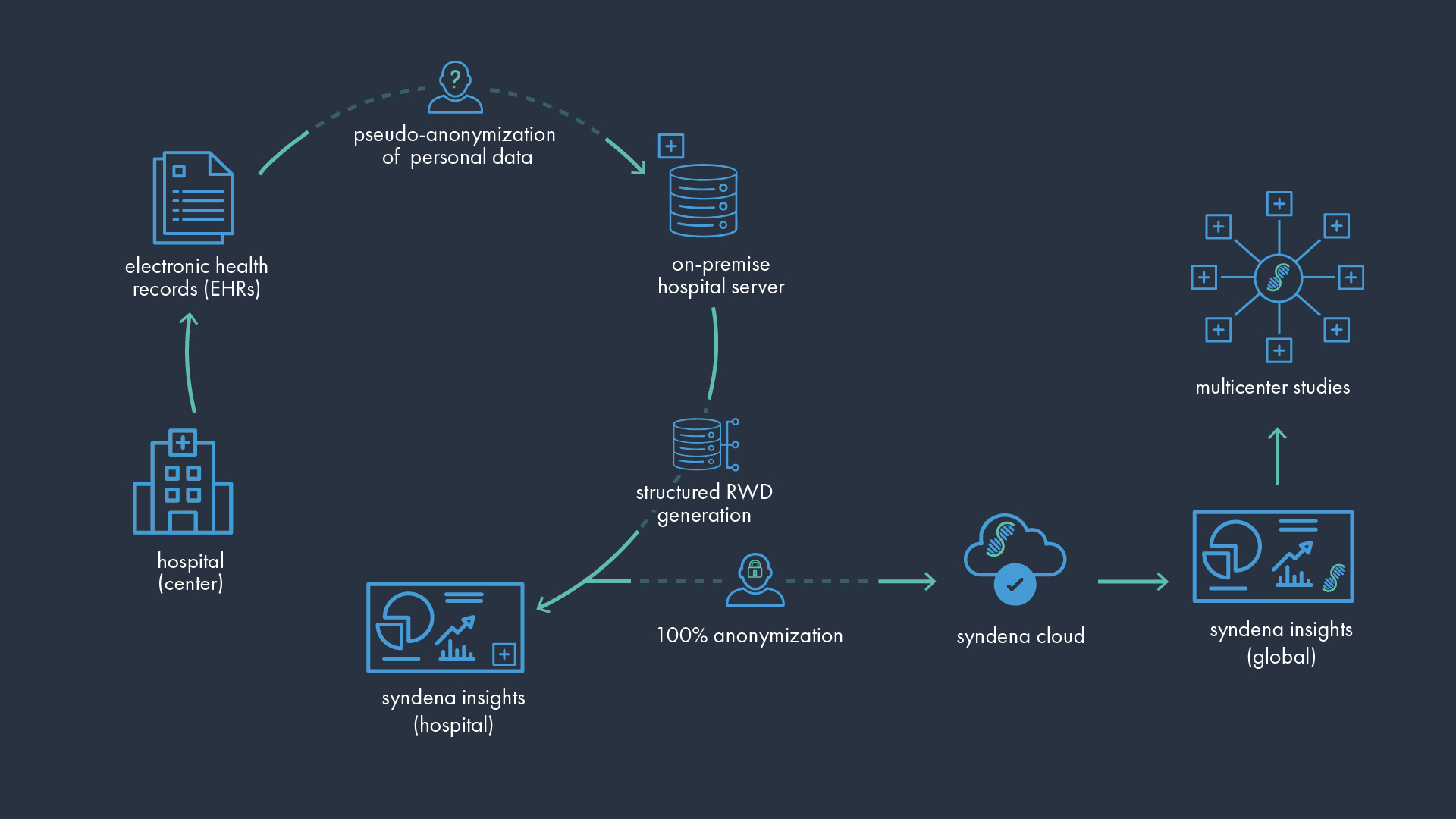
Leveraging AI and NLP, syndena automates the structuring of clinical data by identifying key information in clinical notes uploaded by the clinical teams. The hospitals remain in full control of the patients’ data, while the dashboard supplies life science institutions and research partners with aggregated, anonymised results. The entire process is fully GDPR-compliant, ensuring the highest level of protection and confidentiality for health-related data.
Benefit from a Wide Range of Data Sets
syndena insights hosts a comprehensive repository of patient data, spanning a broad range of indications and incorporating patient reported outcome measures. Beyond mere data, it serves as the cornerstone for informed decision-making, pioneering research and establishing a data-driven healthcare ecosystem.
+ 100,000 anonymised entries of patient data
+ 15 years of follow in multiple indications
+ 6,000 ePROMS
The data from syndena insights can be used especially for the following business units in life science and pharma:
Clinical Trial Design
syndenas utilization of real-world data enhances the precision of clinical trial planning.
Market Access
Our dataset facilitates the launch and development of comprehensive HEOR value dossiers for the life science and pharmaceutical industries.
Medical Affairs
Our dataset enables the extraction of profound insights into clinical outcomes and real-world evidence pertaining to products.
therapeutic areas of sydnena insights.
syndena insights already provides data for a large number of therapeutic areas, and more are being added.
Hematology
Chronic Lymphocytic Leukemia (CLL)
Follicular Lymphoma (FL)
Diffuse Large B-Cell Lymphoma (DLBCL)
Multiple Myeloma (MM)
AL Amyloidosis

Cardiology
(h)ATTR Amyloidosis
Chronic Inflammatory Cardiomyopathy
Gastroenterology
Crohn´s Disease (CD)
Colitis Ulcerosa (CU)
Oncology (Solid Tumors)
Colorectal Cancer

Neurology
Myasthenia Gravis
„For over 10 years, syndena / OncoTyrol has been a highly trusted and recommendable partner, enabling us to deepen our understanding about which treatment trends and which patient outcomes evolved over the years in various real world settings. When you partner with them, their innovative methodologies, along with their scientific approach will lead to excellent project outcomes.“
Uwe Händel, BeiGene, Associate Medical Director Austria
„Syndena works professionally and always adheres to the highest quality standards in implementation. What I particularly appreciate in our collaboration is the pronounced customer orientation and the ability to implement customized projects in a timely manner.“
Bernd Schöpf, Gilead, Associate Director Medical Affairs
“We are extremely pleased with our collaboration with Syndena. Their professional approach and high quality of work have impressed us and exceeded our expectations. The appreciation they have shown for our partnership has contributed to the smooth execution of our projects and the achievement of great results. We look forward to future projects with Syndena and are confident that their expertise and dedication will continue to help us reach our goals.”
Maik Scherholz, Senior Medical Affairs Scientist, malignant Hematology, Pfizer

syndena insights is only one login away
To access the syndena insights global dashboard from the anonymized and real an account with access data.
Let's Start Unleashing the Full Power of Your Data
We’re here to help! We got your back!

syndena – for Data-Driven Treatments in the Healthcare Sector
We firmly believe that data-driven decisions based on real-world evidence drive progress in patient outcomes and scientific research. We are committed to turning this belief into reality. Explore how syndena offers more than just a solution for structuring data.

